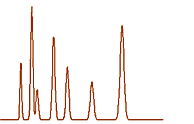

The Pharmakl laboratory has suitable equipment for developing, optimizing and validating of analytical methods for analyses of drugs either in samples of biological origin or in drug formulations for the quality control of pharmaceutical production.
All the work in the analytical laboratory is performed under the GLP (GMP) guidelines as confirmed by relevant certificates. These rules ensure that all operations are made in proper way, by validated methods and are well documented.
Only validated methods are used for sample analyses and quality control program checks the quality and reliability of the results. The analytical methods are not only optimized with respect to sensitivity, precision and accuracy, but also the analysis time is minimized. The analyses of samples from a typical bioequivalence study can be thus completed typically in two weeks after finishing the clinical part of the study which ensures rapid evaluation of the results and final report preparation. The methods which bring a novel solution of the problem are published in scientific literature.